More Clues to the Biology of Alzheimer’s Disease

Alzheimer’s disease remains a puzzle.
Although there are plenty of people who claim that “The Cause” of Alzheimer’s is… and here you can take your pick of three-dozen theories.
The truth of the matter is that simple explanations – Aluminum, lead, stress, pollution, mycoplasma infections and the rest – fail to explain many of the established observations about the causes of the disease.
From what we know about the genes in the brain, it is highly likely that “Alzheimer’s disease” represents a group of disorders that follow an interaction of sets of genetic and environmental factors.
This notion is supported by a report from investigators at Washington University School of Medicine, St. Louis and several other prominent research centers. The study is published in today’s issue of the journal Neuron and indicates that there a biological link between a protein mutation that causes early-onset Alzheimer’s disease (AD) and a gene variant linked to late-onset AD.
The investigators tried to link the function of two known causative factors in AD. Mutations in amyloid precursor protein (APP) cause early-onset Alzheimer’s disease (AD), but
the only genetic risk factor for late-onset AD is the ɛ4 allele of apolipoprotein E (apoE), a major carrier of cholesterol in the circulation and in cell membranes.
Previous research has shown that mutations in APP can cause early-onset AD when the protein is “cleaved” or split, producing a short toxic protein that builds up in the brain and kills neurons.
In their experiments with mice and cultured mouse cells, the researchers linked APP to the regulation of apoE and its cholesterol transport function. They found that the normal cleavage of APP in the cell gives rise to a nontoxic fragment (called AICD) that suppresses the gene that produces the cell receptor for apoE, known as LRP1. Crouching in nerve cell membranes, this receptor enables the apoE protein to transport its cholesterol cargo into the cell.
The researchers speculated that the loss of LRP1 function in AD might cause a loss of cholesterol that causes malfunction of neurons.
These observations may turn out to be extremely important. If we can restore the activity of the receptor gene, we might have a new treatment strategy for AD.
An Antibiotic to Treat Stroke
It is fascinating to see a medicine or a therapy that is used for one thing being applied in a completely different area. A good example is thalidomide, a drug that was introduced in 1957 to treat morning sickness. It was pulled from the market because it could cause terrible fetal malformations. Years later it was discovered that it could be very helpful in the treatment of multiple myeloma, leprosy and Behcet’s disease.
Now a new study has shown that people treated with the antibiotic minocycline within six to 24 hours after a stroke (cerebrovascular accident) had significantly fewer disabilities.
In the study 152 men and women received either an oral dose of minocycline or placebo for five days following stroke. People who received minocycline were treated on average within 13 hours of the onset of the stroke compared to 12 hours for the placebo group. Patients were followed up for three months.
People treated with minocycline had significantly better outcomes than those treated with placebo. By three months, the minocycline group performed four times better than the placebo group on the National Institutes of Health Stroke Scale, which measures vision, facial palsy, movement, and speaking ability.
Minocycline appears to be neuroprotective and to prevent programmed cell death or apoptosis.
This could turn out to be very important because current stroke treatments only work during the first few hours after the onset of symptoms, and many people do not get to the hospital in time to be treated.
Alzheimer’s Disease: Diabetes of the Third Kind?

Insulin may have some extremely important roles in the mind as well as the body.
Though best known for its role in promoting glucose uptake into cells, insulin has at least five hundred known functions in the human body. For many years nobody was sure whether insulin was important to the brain: neurons in the brain are unusual in that they do not need insulin to enter them. But then we discovered that insulin acts on other important metabolic processes in neurons. In the brain it also does duty as a chemical messenger and as a growth factor, and brain insulin signaling is crucial for the creation of memory. Over the years a number of theorists have suggested that some neurological and psychiatric illnesses could be a result of disturbances in insulin-related processes in the brain.
It is now beginning to look as if they may have been correct.
Some recent evidence has suggested that Alzheimer’s disease and diabetes mellitus share a number of common pathways and Alzheimer’s disease is associated with peripheral insulin resistance.
New research has been published by investigators from Northwestern University in the FASEB Journal – the journal of the Federation of American Societies for Experimental Biology – that may finally nail down the reason why insulin signaling may stop working in Alzheimer’s disease.
The research team used mature cultures of neurons from the hippocampus of the brain to study synapses – the connection between neurons – that have been implicated in learning and memory mechanisms. They wanted to examine the effect of a toxic protein, known to attack memory-forming synapses, that is called “ADDL” for “amyloid ß-derived diffusible ligand.” ADDLS are small, soluble aggregated proteins that accumulate in the earliest stages of Alzheimer’s disease. The researchers had previously found that ADDLs bind very specifically at synapses, which in turn initiates deterioration of their function, shape and composition. They now went on and studied the synapses and their insulin receptors before and after ADDLs were introduced. Regions of the nerve with normal numbers of insulin receptors have no ADDL binding, but when the ADDLs are added they remove insulin receptors from nerve cells, effectively making them insulin resistant.
And an insulin resistant neuron cannot participate in the formation of memories.
This finding implies that there is a fundamental connection between diabetes and Alzheimer’s disease, and this may in turn help us to see whether existing diabetes treatments might protect neurons from ADDLs and improve insulin signaling in people with Alzheimer’s disease.
The first type of diabetes is Type 1, a.k.a. insulin-dependent or juvenile onset. The second is Type 2, a.k.a. non-insulin-dependent diabetes. So is Alzheimer’s Type 3?
It has also been known for some time that physical exercise is one of the factors that may help reduce the chance of developing Alzheimer’s disease, and we may now have a mechanism by which it can help.
This is an extraordinarily interesting and important discovery that should lead to a whole new research angle, perhaps with medicines that are already available.
Low Testosterone May Shrink Your… Brain

It is almost forty years since Fernando Nottebohm first began to describe some of the dynamic changes that occur in the brains of songbirds as the seasons change. Every year some regions of the brain grow in response to changes in ambient light levels and others regress. There are marked seasonal changes in the brains of fish, reptiles, amphibians, birds and even some mammals such as gerbils, mice and perhaps even in humans. But the magnitude of the changes in birds far outweighs any other species. It is hoped that that understanding the mechanism controlling that change may help us to develop treatments for age-related degenerative diseases of the brain such as Parkinson’s and dementia.
Researchers from the University of Washington and the University of California, Berkeley, have published some interesting new data in the Proceedings of the National Academy of Sciences. They report a striking shrinkage in the size of the brain regions that control singing behavior of Gambel’s white-crowned sparrows. This transformation is triggered by the withdrawal of testosterone and can be seen within 12 hours. The study is the first to report such rapid regression of brain nuclei caused by the withdrawal of a hormone and a change in daylight conditions in adult animals.
The research protocol was designed to mimic the natural seasonal changes that occur in the brains of the sparrows. Their song-control regions expand in the spring and summer leading up to the breeding season, as they use songs to establish territories and attract mates in Alaska. Later in the summer, as the birds get ready to migrate back to California, the same brain regions shrink.
To better understand what happens in the sparrows’ brain, the researchers received federal and state permits to capture 25 of the migrating male birds in Eastern Washington. They then housed the birds for 12 weeks before exposing them to 20 days of long-day conditions comparable to the natural lighting the sparrows would experience in Alaska during the breeding season. The birds were also implanted with testosterone.
At the end of 20 days, six of the birds were euthanized and the remaining 19 were castrated and testosterone implants were removed so there would not be any circulating testosterone in their systems. After 12 hours five more birds were euthanized and the remainder were euthanized at 2, 4, 7 and 20 days.
The researchers found that the size of the high vocal center (HVC) region decreased 22 percent within 12 hours after the withdrawal of testosterone and that the number of neurons in this song-control region fell by 26 percent after four days. In addition, the size of two other song-control regions called Area X and the RA significantly regressed after 7 and 20 days.
Much as I dislike animal experiments of any kind, it is invaluable to have an animal model system in which we can observe predictable neurodegeneration. As men age, circulating levels of testosterone decrease, and other research has shown that this decline may contribute to cognitive impairment.
This is an important new approach to understanding the interplay between nerve cell degeneration and hormones.
Even Neurons Need Friends

In many neurological diseases, including multiple sclerosis (MS) and some types of neuropathy, myelin – the insulation that covers and surrounds most of the larger nerves – is damaged or deranged.
Scientists at the Weizmann Institute of Science in Rehovot, Israel, working with colleagues in the United States, recently reported finding an important new line of communication between cells in the nervous system that is crucial to the development of myelinated nerves. This new discovery may eventually help us to restore the normal function of the affected nerve fibers.
Nerve cells (neurons) have long, thin extensions called axons. The longest of these axons is in the verves supplying the feet, and they can be over three feet or more in length. The larger axons are covered by myelin, which is formed by a group of specialized cells called glia.
Glial cells move around the axon, laying down the myelin sheath in segments, while leaving small nodes of exposed nerve in between. Myelin provides protection for the delicate axons, and it also allows nerve signals to jump rapidly between the gaps or nodes, increasing the speed and efficiency of the transfer of electrical signals down the axon. When myelin is missing or damaged, the nerve signals cannot skip down the axons, leading to abnormal function of the affected nerve and eventually the now naked nerve may degenerate and die.
In research published in Nature Neuroscience, Elior Peles, Ivo Spiegel and their colleagues in the Molecular Cell Biology Department at the Weizmann Institute and in the United States, provided a new insight into the mechanism by which glial cells recognize and myelinate axons.
They found a pair of proteins that pass messages from axons to glial cells. These proteins, called Necl1 and Necl4, belong to a larger family of cell adhesion molecules. The whole class of cell adhesion molecules does exactly that: they sit on the outer membranes of cells and provide the glue that helps them to stick together. Even when removed from their cells, Necl1, which is normally found on the surface of the axon, and Necl4, that is found on the glial cell membrane, adhere together very tightly. When these molecules are in their natural homes of the axon and glial cell, they not only create physical contact between axon and glial cell, but also serve to transfer signals to the interior of the glial cells, initiating changes needed to undertake myelination.
The scientists found that production of Necl4 in the glial cells rises when they come into close contact with an unmyelinated axon and as the process of myelination begins. If Necl4 is absent from the glial cells, or if they blocked the attachment of Necl4 to Necl1, the axons that were in contact with the glial cells did not myelinate.
This is an exceptionally important discovery. Most of the approaches that we use for treating MS, peripheral neuropathy and other degenerative diseases, can often help with symptoms and may slow but not cure the diseases. But if we can understand the mechanisms that control the process of wrapping the axons by their protective sheath, we may be able to recreate that process in patients.
A New Treatment for Parkinson’s Disease?

It is remarkable how often we find that a treatment for one problem may turn out to help something completely different.
Parkinson’s disease is caused by a loss of dopamine neurons in specific circuits in the brain. Dopamine has a great many actions, but I these particular circuits it is involved in the control of movement. Once it starts, the process usually continues on inexorably. The mystery has been why the neurons die in the first place.
There is a very promising article that was published online today in the journal Nature.
Investigators from the Department of Physiology in the Feinberg School of Medicine at Northwestern University in Chicago and the Department of Cellular and Molecular Pharmacology at the Chicago Medical School, Rosalind Franklin University of Medicine and Science have looked at a medicine that is usually used for treating hypertension, and found that it may protect dopaminergic neurons.
Their studies have suggested that the dopaminergic neurons involved are unusually reliant on a type of calcium channel that maintains their rhythmic activity. If the pacemaker is damaged, the neurons become more vulnerable to stressors such as neurotoxins. As we age, calcium ions begin to enter the neurons and change their behavior. They showed that mice that had been engineered to develop a progressive Parkinson’s-type disease did not become ill when their condition was treated with the calcium channel blocker israpidine. Their dopamine neurons appeared to revert back to their original, youthful form.
It is much to early to think about using isradipine for treatment. We do not, for example, know whether it works in humans and we have any idea how much of the drug would be needed. But this research does suggest a whole new line of research and possibilities for treatment.
That is exciting, though when I read about research like this I always wish that researchers did not use animals in their experiments. But since the animals have made the sacrifice to help us, we need to acknowledge their help.
Alzheimer’s: “A Disease of Civilization?”
I have had the pleasure of receiving some excellent comments after the recent article about Alzheimer’s disease.
One suggestion was that Alzheimer’s is a modern illness caused by mercury in dental fillings. This has been a popular idea for several years, and I knew an holistic dentist in Philadelphia who told me about some people who seemed to be relieved of an array of symptoms after their mercury-containing fillings were removed.
Over the last few years I have spent a long time reading books on the topics and analyzing published studies in detail.
These are some of the books with which I started:
- Warren T. Beating Alzheimer’s: A Step Towards Unlocking the Mysteries of Brain Diseases. Garden City Park, New York: Avery Publishing Group Inc., 1991.
- Huggins HA. It’s All in Your Head: The Link Between Mercury Amalgams and Illness. Garden City Park, New York: Avery Publishing Group Inc., 1993.
- Walker M, Whitaker J. Elements of Danger: Protect Yourself Against the Hazards of Modern Dentistry. Charlottesville, Virginia: Hampton Roads Publishing Company Inc., 2000.
- Hardy JE. Mercury Free: The Wisdom Behind the Global Consumer Movement to Ban "Silver" Dental Fillings Glassboro, New Jersey: Gabriel Rose Press Inc., 1996.
One of the best resources that I’ve found for people interested in the potential problems that may be caused by dental amalgams is here.
Clearly there is a potential for harm. At one time I considered having my fillings removed, but in the end I didn’t.
Why did I make that decision?
After all, mercury is neurotoxic, which is one of the reasons for all the concern about thimerosal in vaccines, and its alleged link to autism. And I would prefer to avoid getting Alzheimer’s disease. I have no family history of it. In fact the opposite: my father was estimated to have an IQ in the 170-180 range when he was in his nineties. But it is still a concern.
While some people seem to have benefited form having their fillings removed, many have not. So there must be some other factor or factors, likely including a genetic predisposition. People have created genetically deformed animals with all the signs of Alzheimer’s disease, but without a molecule of mercury in sight.
It is constantly being said that Alzheimer’s disease is a modern illness, and that its appearance roughly coincided with the introduction of mercury fillings. But that’s not true. Alois Alzheimer did describe the classic pathological signs of the disease in 1906. It was only made possible by the development of a new silver staining method developed by Alzheimer’s colleague Franz Nissl. For the first 70 years after it was first described, Alzheimer’s disease was regarded as a “Pre-senile” dementia.
But dementia had been described at least two thousand years earlier by Lucretius.
Some of those early descriptions probably included a ragbag of different illnesses, but for at least 2,000 years, from Rome to China, there were clear descriptions of something that looks just like modern Alzheimer’s disease. There are descriptions by the great English physician Thomas Willis, and the term “demented” entered the English language in 1644. There was a famous description of Sir John Roberts of Bromley, who was described by his physician, William Salmon as “decayed in his intellectuals.” That was in 1694.
There are extensive descriptions of “senile dementia” from many of the famous names in the history of psychiatry, including Cullen, Pinel and Esquirol. So the idea that it is a modern illness is not correct. Has it been becoming more common? Possibly, but it is difficult to say, because in 1905 the average life expectancy in the United States was 47 years.
When I really started analyzing the research in detail, I was not impressed by the data linking mercury with Alzheimer’s disease and multiple sclerosis. There are definitely some reports (1.2.) of possible links between neurodegeneration and mercury, but the most recent epidemiological studies have failed to find a link. Indeed there has been a great deal of research indicating that – for most people – amalgams are safe. ( 1. 2. 3. 4.) although it is important that the research should continue. Last year the U.S. Food and Drug Administration (FDA) panel called for an additional review of scientific studies on the safety of dental amalgam fillings. Some of the responses are here.
My own take on this is as follows:
- The evidence for a causal link between mercury – primarily from fillings – and Alzheimer’s disease is thin.
- The quality of the evidence for many other conditions, such as multiple sclerosis is also not of good quality.
- There is still a great deal that we do not know about the medical consequences of mercury containing amalgams, particularly in children and pregnant women.
- I am going to continue to update this information as more data is published.
At the moment I still have my fillings.
But there is another reason: the few that I have were done in England, and for years now there have been restrictions on the use of mercury-containing amalgams.
Lithium and Brain Growth
There’s an interesting story about the way that lithium was found to be helpful to some people with bipolar disorder. It was thought that there was a link between bipolar disorder and gout, and gout is caused by an accumulation of uric acid in some joints. It just so happens that lithium is very good at getting guinea pigs to pass any excess uric acid in their urine. So in 1949 a medical scientist in Australia called John Cade tried lithium in bipolar disorder. It worked, but not because of any effect on uric acid: the lithium/uric acid connection only works in guinea pigs. But now, all these years later, it turns out that there may actually be a link between bipolar disorder and uric acid after all: uric acid levels often rise in people who are insulin resistant, and insulin resistance is a common feature of bipolar disorder.
The last few years have seen the growth of an impressive body of evidence about the effects of lithium on the brain. Lithium modulates the way in which many neurotransmitters act in the brain. That raised interesting questions, because many of the chemical transmitters do not only send messages between neurons, they may also stimulate the growth of cell processes and perhaps neurons themselves. Then it was discovered that lithium protects neurons by increasing the levels of a neuroprotective protein called Bcl-2. Then it was found to help stimulate the production of new neurons (neurogenesis) in the hippocampus.
A major breakthrough came in 2000, with the demonstration that lithium increases the amount of gray matter in the human brain, probably by stimulating the production of a growth promoter called brain-derived neurotrophic factor. Gray matter is composed primarily of neurons, so this suggested that lithium was either stimulating the growth of new neurons, or causing the migration of neural stem cells, or alternatively somehow preventing the death of neurons.
Within less than two years it was confirmed that treatment with lithium increased the amount of gray matter in the brains of people with bipolar disorder.
The findings remained controversial for two reasons: first was the seeming impossibility of stimulating the production of new gray matter. That is no longer such a problem: there is more and more evidence that the adult brain can indeed produce new neurons.
The second problem was technology: the measurements were open to
different interpretations.
Now neuroscientists at UCLA, using brain scans collected by collaborators at the University of Pittsburgh School of Medicine have shown that lithium does indeed increase the amount of gray matter in the brains of patients with bipolar disorder. But what is far more important is that the effects are seen in precise regions of the brain that are thought to be implicated in bipolar disorder.
The research is published in the July issue of the journal Biological Psychiatry.
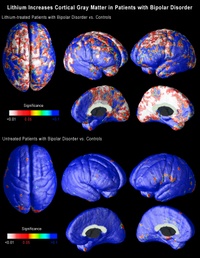
Carrie Bearden, a clinical neuropsychologist and assistant professor of psychiatry at UCLA, and Paul Thompson, who is associate professor of neurology at the UCLA Laboratory of NeuroImaging, used a novel method of three-dimensional magnetic resonance imaging (MRI) to map the entire surface of the brain in people diagnosed with bipolar disorder. Everyone interested in the brain has been amazed by the quality of the images coming from the UCLA lab over the last six or seven years. (Graphics from the study, including the one above, are available online here.)
When the researchers compared the brains of bipolar patients on lithium with those of people without the disorder and those of bipolar patients not on lithium, they found that the volume of gray matter in the brains of those on lithium was as much as 15 percent higher in the cingulate and paralimbic regions of the brain, that are critical for attention, motivation and emotional control. There was no change in the amount of white matter, telling us that these effects are unlikely to be either artifacts or swelling of the brain.
These are extraordinary findings that may help explain how lithium works. If lithium increases the amount of gray matter in specific regions of the brain, this may suggest that existing gray matter in these regions may be underused or dysfunctional in people with bipolar disorder.
Conventional MRI studies in bipolar disorder measured the total volume of the brain, but these new imaging techniques employ high-resolution MRI and cortical pattern-matching methods to map gray matter differences that are so precise that they can pick up subtle differences in brain structure and this is the first time that researchers have been able to look at specific regions of the brain in people with bipolar disorder, and examine the effects of treatment.
The researchers at UCLA analyzed MRI scans of 28 adults with bipolar disorder, 20 of whom were lithium-treated, and 28 healthy control subjects collected by collaborators at the University of Pittsburgh. The analysis involved detailed spatial analyses of the distribution of gray matter by measuring local volumes of gray matter at thousands of locations in the brain.
This is superb research, and one of the signs of good research is that it raises yet more questions:
- We do not know whether these effects on gray matter persist once the medicine is stopped.
- Neither do we know whether the same effect might occur in other illnesses in which there is loss of gray matter. It may just be that lithium corrects an abnormality that is specific to bipolar disorder. That being said, there is recent evidence that lithium may be neuroprotective in a mouse model of Alzheimer’s disease.
- We also do not know if these brain changes correlate with clinical improvement.
- Lithium can have a lot of side effects, so we shall also need to factor that into the equation.
- We also need to know whether other medicines – or any other kind of therapy – might produce similar effects: there are already several medicines that have been shown to do something similar in some animals.
It’s still a bit too early to suggest putting lithium in the water supply…
Food Additives and Behavior

Few things generate as much heat and as little light as the debate about a possible association between food additives and cognition, mood and behavior.
There are a number of ways in which food may influence all three, including:
- Malnutrition
- Composition of the diet
- Nutrient quality of the diet
- Eating habits
- Pharmacological effects of foods
- Food allergy
- Food sensitivity
- Contamination of food with heavy metals, hormones and pesticides
- Fatty acid deficiency
- Food additives
It is often surprising to learn that many people do not realize that in children – particularly if malnourished – omitting breakfast can have a marked effect on cognitive functioning. But it is the last of those that I want to look at today.
Until the 1950s if food manufacturers wanted to add color to a food it was done primarily with natural plant and vegetable based compounds: pale red colors could be achieved from beets; green from chlorophyll-containing vegetables; yellows and orange could be achieved from extracts from a number of other plants and spices. But then things began rapidly to change as we outlined in Healing, Meaning and Purpose.
The notion that food additives could be a cause of hyperactivity is at least 30 years old. I think that Ben Feingold was the first to introduce the idea and with it his notoriously difficult diet.
Over the years there have been some positive clinical trials of the diet and some negative. But I think that every clinician working with behavior problems has seen some startling improvements in some children and adolescents when they go on an elimination diet.
In 1985 a controversial study published in the Lancet claimed to show that 79% of hyperactive children had symptomatic improvement when food chemicals were removed from their diet. Then when the food chemicals were re-introduced the symptoms returned. No other study has ever produced figures anything like that high.
It is also important that in young children, though additives may cause a problem in some, there does not seem to be a link between allergies and food sensitivities, and parents often pick up behavior changes that simple clinical screening tools do not. So mom and dad may really know best.
Several years ago we tried to look at the impact of food additive not on behavior, but on headache. When the additives were administered double blind, we were unable to replicate most people’s symptoms, even when they were sure that a certain food caused a problem.
However, unsupervised restriction diets are not without their dangers. And we also need to make sure that practitioners know what they are doing: I once saw a young woman who had seen by an “alternative allergist,” who had left her on a diet consisting of spring water, rice and lettuce. And nothing else.
Another problem is that many of us do not know what additives are lurking in the food that we eat. There was a recent study in the United Kingdom indicated that on average, Britons consume 20 different food additives every day, with some eating up to 50. Yet most people were unaware of this figure, with nearly half of the 1,006 people surveyed thinking they ate only 10 additives each day.
The research also found that many people did not understand which foods are most likely to contain additives. I have not yet seen the raw data from this study, but I shall have more to say about it once it becomes available.
A number of large independent studies are currently underway (for example, here) which should help us to better identify who is susceptible to additives, how to test for sensitivity to additives and who might benefit from their withdrawal.
The trouble with a lot of the discussion about food additives, behavior, mood and cognition is that it usually begins from a false premise: that there is a single cause for a behavior.
When I am teaching it continues to astonish me that most health care professionals still expect there to be one “cause” for a problem. Yet as I have mentioned before, this is rarely clinical reality.
A food additive may be associated with problems, but only in a minority of children, and only if they are genetically predisposed and if the right set of environmental circumstances are in place.
If you suspect a problem, first, learn to look at labels. And see if simple exclusion helps. An allergist is the next stop, but also ask whether an additive could be causing a biochemical rather than an allergic problem. If it is a biochemical effect, it may not show up on routine allergy tests, but there are other ways of testing.






